7 min read

Cloud computing
Cloud computing is quickly becoming more and more popular among biotech and pharma companies. If the pace of migration into the cloud remains at the present level, we will witness a revolutionary improvement in medicine development.

Thanks to cloud computing, the odds of developing new drugs faster and cheaper have drastically increased. It is no wonder that pharma and biotech companies migrate to the cloud, seeing it as a solution to many of their existing problems like the increasing pace of work, data analysis, or cooperation. With next-generation technologies, such as artificial intelligence and machine learning, the cloud enables acceleration for the entire industry. The lion’s share of success lies in the rapid collection and analysis of crucial data. Efficiency in that area, the shortening of drug discovery processes or trial tests, and lowering costs seem to be significant factors in a company’s growth.
Cloud computing is not just infrastructure — it’s an evolution of software architecture. The last 20 years of such evolution gave us reusable assets that now are part of the cloud DNA — modularity and the rise of managed services. Nowadays, clouds come with a toolbox of services that greatly speeds up development and shortens the time to market.

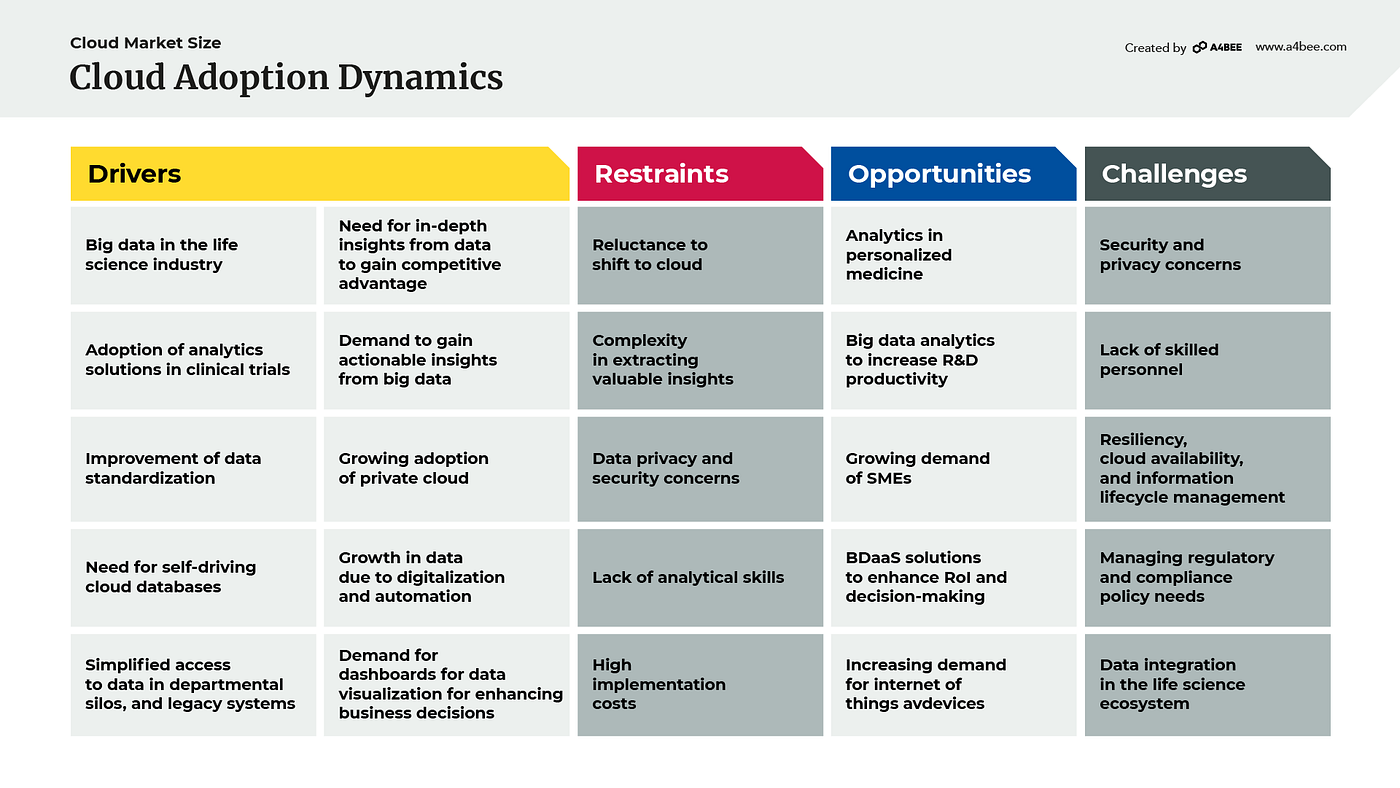
Cloud adoption dynamics
Until recently, migration to the cloud by regulated industries wasn’t as common as in the non-regulated market. Cloud providers have quickly come to understand that they need to answer the needs of the life-science industry. At first, many biotech companies expressed concern, but soon the migration of almost the whole industry became a reality. Covid-19, with its urgent necessity of finding a cure or vaccines, or remote access to manufacturing sites, has contributed to speeding up this process.
Cloud adoption — Biotech case studies
We’ve collected examples of implementations where you can see benefits across the value chain — from the R&D area to compliance. Cloud computing has become the most efficient technology for biotechnology and pharmaceutical companies’ everyday research, manufacturing, and distribution challenges.
Research & Discovery
Discovery and preclinical research in bio-pharma manufacturing benefit the most from cloud solutions, with 60% of the cloud computing market (source: BCC Research Cloud Computing in Cell Biology, Genomics and Drug Development). Moderna is an essential player in the biopharma industry but is a perfect example of how harnessing the cloud accelerates a company. It delivered the first clinical batch only 42 days after the initial sequencing of the COVID virus. This accomplishment was possible thanks to building and scaling all operations using cloud and cloud-based solutions. The first doses of the Covid-19 vaccine, Moderna, were delivered to the National Institutes of Health in February 2020. Moderna’s scientists used cloud-based methods and prototyping inventions to rapidly experiment with reading the virus’s mRNA to accomplish this feat. They could quickly shift between analyzing and creating vaccines for different viruses while using the same infrastructure. Two years later, Moderna’s leading position is stable, and we can be assured the company will harness its experience in future drug and vaccine inventions. While they worked on “mRNA as a bio platform” for over a decade, cloud data enablement and analysis gave them the chance to act quickly and highly effectively. The story of Moderna shows its unique way of using cloud computing for a spectacular finish of its long-term research and discovery.
Six years ago, we started building databases and information-based activities to support all our programs. Today, we’re fully cloud-based, and our scientists don’t go to the lab to pipette their messenger RNA and proteins — they go to our web portal, the Drug Design Studio that sits in the AWS Cloud. […] Over the years, data from the portal and lab has helped us improve our sequence design and production processes and improve the way our scientists gather feedback. Our data scientists built algorithms to accelerate the design of sequences for messenger RNA. In terms of research, all our algorithms rely on computational power from AWS to further our science…
– Marcello Damiani, Moderna
Read the whole conversation here.
A similar approach helps many other companies to see benefits in cloud-based analytical technology and the optimization of clinical research, development, and manufacturing.

Supply Chain
Everyone can imagine how consequences can bring supply chain problems between drugmakers and patients. To prevent that, many companies harness cloud computing into products and supply transfer processes. One of them is Novartis. Novartis created a whole new pipeline system to monitor every step in the partnership chain with Amazon. The backbones of this process are ‘Insight Centres’, which maintain complete and real-time visibility among manufacturer and distributor centres.
The company gathers inventory, quality, and production data across its network and applies emerging technologies like the Internet of Things (IoT) and machine learning (ML) services to gain broader visibility. It enables Novartis’ manufacturing and planning teams to precisely forecast and track production lines, detect bottlenecks and make adjustments to improve accuracy.
By linking all operations with actual data, planning and potential forecasting risks are much more efficient than ever before.

Monitoring
Bayer AG develops the Crop Protection Innovation Lab as a service for agriculture. The Innovation Lab designed and developed a low-cost Internet of Things (IoT) pest-monitoring device. The system alerts farmers if a pest migration happens in their fields, enabling them to take early action to protect their crops. The device takes multiple pictures per day and uses image recognition to identify pests. It delivers results on a mobile application on farmers’ smartphones within minutes. The application provides farmers guidance on the protection actions that they should take. Thanks to the system, farmers can prevent damages.
Furthermore, scalable serverless architecture on AWS makes the system accessible and egalitarian by cutting costs for operation by 94 per cent.
As a company committed to innovation in agriculture, we lacked the real-time data collection and analysis needed to catch any issues with equipment calibration, jamming, or deviations to help with routing plans for subsequent runs. […] In our seed business, it’s all about getting better and faster visibility into what’s going on in the fields during planting and harvest within our breeding research and supply chain organizations. Using AWS IoT, we can get seed data to analysts in just a few minutes instead of a few days.
– Peri Subrahmanya, Product Manager, IoT, Bayer Crop Science
Read the whole case studies here.
With minimum effort and a few lines of code, we were able to solve the major challenge of providing cost transparency and traceability. This gives us a solid foundation for future infrastructure optimization.
– Dr Alexander Roth, Head of Engineering, Crop Protection Innovation Lab, Bayer AG
Read the whole case studies here.
Compliance
Estimates of R&D costs only per new medicine ranged from US$944m to US$2,826m. But it takes much more to bring a new drug to the market. The validation process can get complicated and costly. Cloud-based quality and compliance management tools can streamline the process of changing and updating the system and make information available faster.
CAKE — the Change Assessment Knowledge Engine is Merck’s answer to the question of how to benefit from cloud-based platforms in the compliance area. Using cloud-native services such as AWS Neptune graph database and advanced ML algorithms (NLP), CAKE provides a high-quality process of updating and listing all regulations relevant to the biotechnology and pharmaceutical industry. The CAKE system generates recommendations for the scientist on regulatory impact reporting and accompanying hyperlinks to specific rules. It speeds up implementation and guarantees compliance from beginning to end in R&D, production, and delivery processes.
The top biotech software is already cloud-based
Cloud adoption is not limited to infrastructure or custom development applications. Vendors providing out-of-the-box digital biotech & manufacturing offer them in the form of cloud deployment models. Process Historian, MES, LIMS or EBR — there are tools available in cloud deployment models in those categories. Vendors leverage all three cloud deployment models: public cloud, hybrid cloud, and private cloud.
Looking at the Laboratory Information Management Systems market, the LIMS market reached 0.8 mln EUR in 2020 and will grow by 12% CARG up to 1.4 mln EUR in 2025 (BCC Research Cloud Computing in Cell Biology, Genomics and Drug Development). Cloud-based LIMS growth rate is even faster. As of 2020, LIMS software solutions available in the on-premise deployment model — made up 53% of the overall market, and those available on public cloud-only 28%, both options 19%.

Many well-known LIMS systems like Sample Manager from Thermo Fisher, Starlims from ABBOTT, Agilent SLIMS, LABWare LIMS are available in cloud deployment, both public and hybrid cloud (and on-premise too). More and more vendors offer cloud-based solutions in the LIMS space, including new emerging players entering the market.
The evolution of cloud computing shows how scientific development can be extensive and complex, and that is excellent news for the whole industry and every person in the world. Looking at cloud users’ stories, we receive glimpses of our future — where personal treatment is fast and available, our environment is protected, and new solutions are accessible to everyone, not only to the richest.
Novartis, Bayer, Merck, and other companies take advantage of cloud computing, but each of them makes it on their terms. They demand individual approaches from their providers and use existing opportunities as much as possible. By looking at their engagement and successes, we can easily discern lessons for ourselves. Change is good; change while using flexible and well-suited solutions is even better.






