5 min read

Introduction
One of the key principles of the Secure Product Development Framework (SPDF) is Security by Design. This concept, which is essential from a cybersecurity standpoint, is especially relevant to medical devices in the context of FDA guidelines. In this article, You will find key standards used to build a framework for securing industrial automation and control systems (IACS), outlining specific system requirements (SR) for larger setups and component specific requirements (CR) for device producers. Pharma specific hardware and software development is far more complex than only Cybersecurity, but securely designed Product may address safety aspects too.
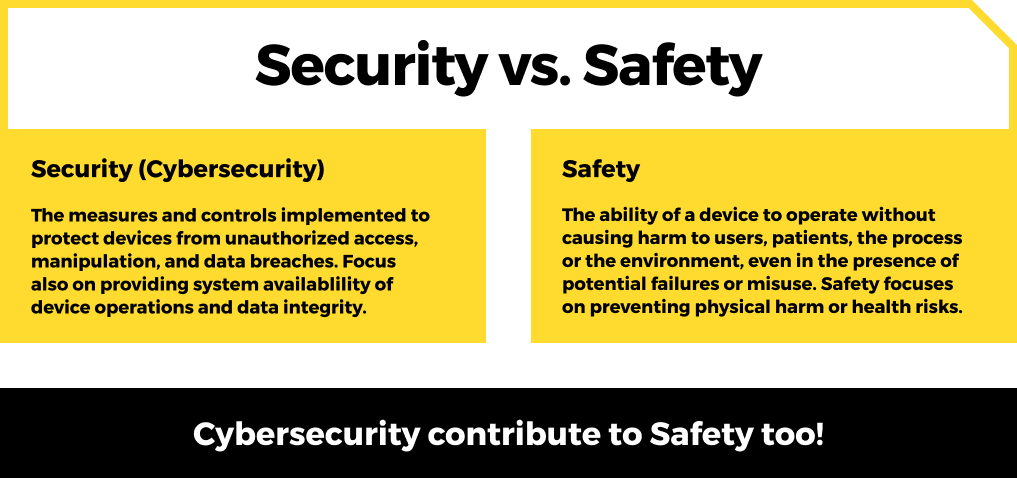
Security vs. Safety
Let’s clarify important terms. I’m sure it’s not necessary to explain it for everyone, and all of you know the difference between the terms security and safety. However, it might be confusing to hear those in some contexts. Although they are very similar terms, they touch pretty much different topics.
Secure Product Development Framework
A Secure Product Development Framework (SPDF) is a structured approach that integrates security considerations throughout the entire lifecycle of a product, from its initial design to its eventual decommissioning. In the context of Industrial Automation and Control Systems (IACS), especially within the Life Sciences, a robust SPDF is crucial to ensure that devices and systems are secure, reliable, and capable of protecting data integrity and patient safety. The life sciences industry relies heavily on interconnected medical and laboratory IoT devices. These devices often handle sensitive patient data, and their functionality is critical for diagnostics, treatment, and important scientific research. Cybersecurity breaches can have severe consequences, including compromised patient data, disrupted operations, and even patient harm. The FDA recognizes the critical importance of cybersecurity in medical devices and promotes the use of SPDFs to ensure devices are designed and operated securely.
A well-structured SPDF is essential for several reasons:
● Reducing Vulnerabilities: An SPDF helps to identify and mitigate vulnerabilities throughout the product lifecycle, reducing the risk of successful cyberattacks.
● Ensuring Data Integrity and Confidentiality: By incorporating security controls and best practices from the outset, an SPDF helps protect sensitive data from unauthorized access and tampering.
● Meeting Regulatory Requirements: In the life sciences, compliance with regulations such as FDA guidelines is critical. An SPDF can help manufacturers meet these requirements by demonstrating a proactive approach to cybersecurity.
● Improving Efficiency: By integrating security into the development process, an SPDF can prevent costly re-engineering of devices and systems later, saving time and resources.
● Supporting Supply Chain Security:The SPDF also helps ensure that third-party components, including open source software, are properly vetted for vulnerabilities throughout their lifecycle

System-level and Component-level Cybersecurity Requirements
The IEC 62443 standards are structured around seven Foundational Requirements (FRs) that serve as the cornerstone for both system-level and component-level security. These FRs provide a comprehensive framework for addressing different aspects of cybersecurity within industrial automation and control systems (IACS)
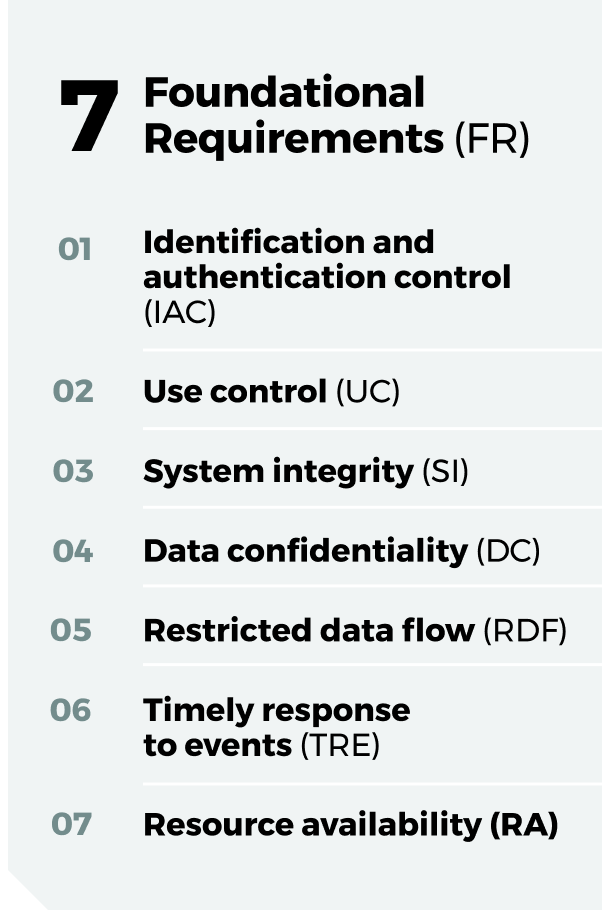
The principle of “Security by Design” is a proactive approach that integrates security considerations from the initial stages of a system’s or component’s development lifecycle. Rather than treating security as an add-on, it becomes a fundamental aspect of the design process. This is particularly crucial in the realm of industrial automation and control systems (IACS), where vulnerabilities can lead to significant health, safety, environmental, and financial consequences. The IEC 62443 series of standards provides a comprehensive framework for implementing “Security by Design” at both system and component levels.
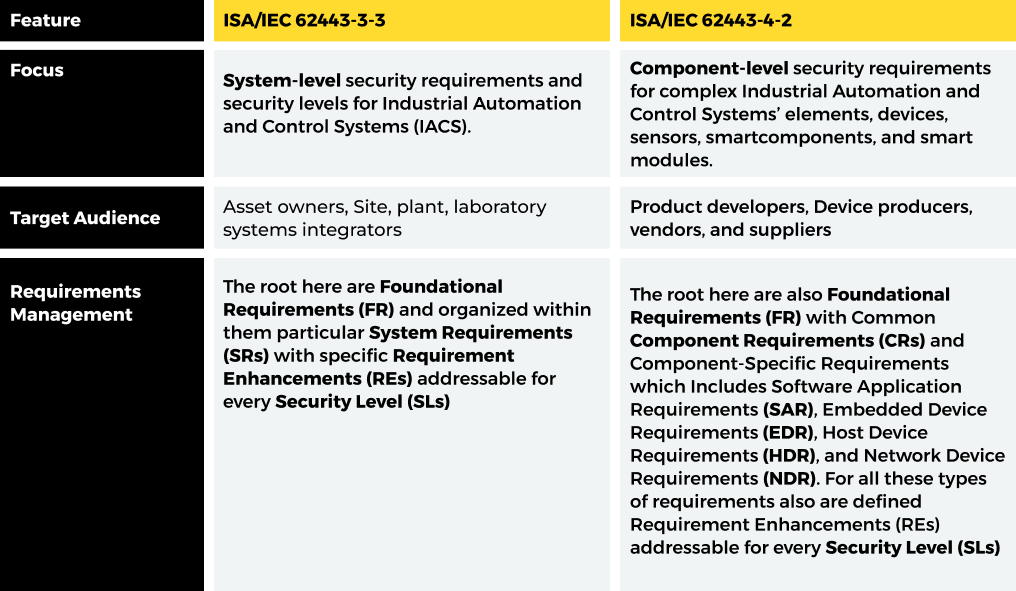
In summary, IEC 62443-3-3 focuses on system-level requirements and defining security levels for control systems, while IEC 62443-4-2 focuses on component-level requirements, deriving them from IEC 62443-3-3, and specifying how to achieve security levels in individual components. IEC 62443-4-2 is more specific, dealing with particular types of components like embedded devices or network devices.
Cybersecurity in devices for LifeScience
Cybersecurity is not just an IT concern but a critical element for safeguarding the efficacy and safety of devices in the life science and healthcare industries. Given the increasing ophistication of cyber threats and the growing reliance on interconnected medical and laboratory devices, a robust approach to cybersecurity is essential. This approach must encompass both system-level and component-level security, along with a well tuned Secure Product Development Framework (SPDF). Holistic approach to cybersecurity is a rule #1. It must be a collective responsibility, permeating every facet of an development process. Inter-departmental collaboration is vital for a robust security framework, enhancing not only data and communication protection but also the organization’s reputation when product is released to the market. Continuous monitoring and adaptation of SPDF to fast changing surface of threats worldwide (e.g. emerging A.I. solutions adoption) must be integral part for continuous implementation and improvement of every organization.
Conclusion
Implementing the Secure Product Development Framework and its “Security by Design” principle is a game-changer for ensuring the cybersecurity of industrial automation and control systems. By integrating security into every aspect of development—from system architecture to component design—organizations can significantly reduce vulnerabilities, enhance reliability, and build trust. The IEC 62443 standards provide a framework focused on moving security from an afterthought to a core part of development practices. It is worth to highlight the importance of a Secure Product Development Framework (SPDF) and its relevance to regulatory compliance goals. Particularly concerning is the EU’s Cybersecurity Resilience Act (CRA) and its applicability to the device vendors in EU market also in Life Science and Healthcare.
See also article: Cybersecurity: Building Resilience Against Anomalies






