5 min read

The Role of Robots in the Evolving Biotechnology and Pharmaceutical Industry
Today’s biotechnology and pharmaceutical industry is setting increasingly ambitious targets for low-volume production. Pursuing these goals is driven primarily by the need to increase flexibility and speed of response to changing market conditions and demand for specific products such as individualized therapies.
Automation of short-run production is also essential for R&D laboratories, where typical high-volume machines are not applicable, and collaborative robots are gaining popularity. Automating processes with robots is a crucial tool for achieving desired goals. Through the use of advanced technology, pharmaceutical manufacturers can reduce production time, minimize waste, and increase product availability for patients.
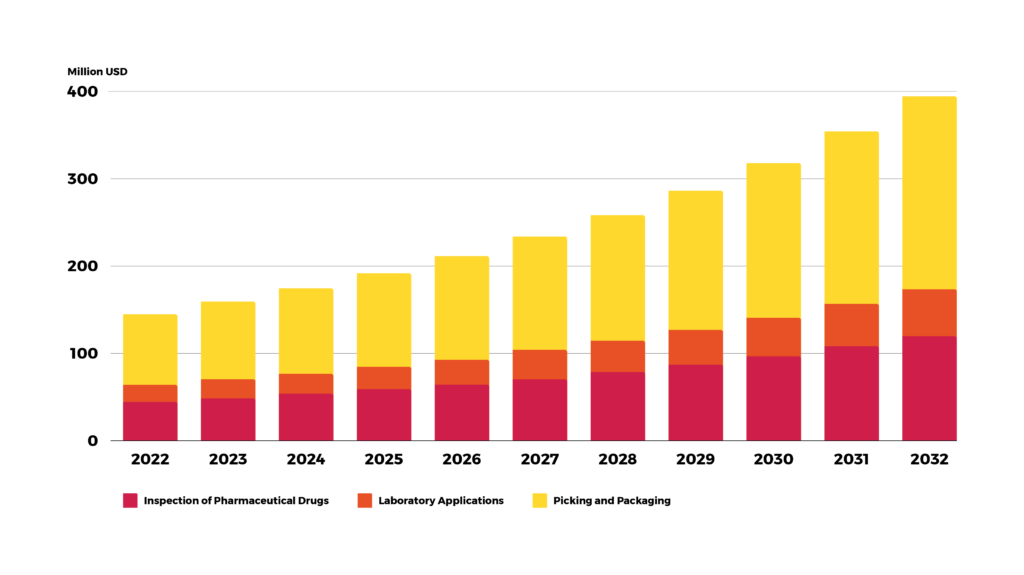
Use of robots in cleanrooms
Robots are not only used for typical applications , such as packaging or palletizing and warehouse use. They also significantly impact cleanrooms, save space, and enhance safety. Multipurpose robots are becoming standard for interleaving (pick and place) operations and opening, filling, and closing in small-volume production.
Using robots to handle chemical and biological substances hazardous to humans is a game-changer, increasing the safety of workers, reducing the risk of accidents and occupational diseases, and ensuring a reduced risk of contamination of the zone by minimizing the necessary intervention of personnel.
Maintaining repeatability and quality
One of the critical aspects of pharmaceutical manufacturing is maintaining repeatability and product quality. Using robots in production processes makes it possible to eliminate human error and ensure consistent product quality by performing tasks accurately and reproducibly.
Operations performed in an automated manner archived by regulations make it easy to find the causes of non-conformities, which can be discovered after products are placed on the market. Increasingly widespread integration with MES and ERP systems allows for quick and error-free retrieval of information about the type of product and automatic change of the recipe, which makes it possible to reduce the time required for machine changeovers. Collected quality data – information on the type of rejects, filling level, recorded images of analysis, etc., allow quick and accurate response in case of quality problems.
Maintaining sanitary standards
In the pharmaceutical industry, where products are intended for direct consumption or human use, maintaining the highest sanitary standards is not just important; it’s crucial.
Robots can operate under controlled environmental conditions, minimizing the risk of contamination of products by microorganisms or other undesirable substances. Manufacturers are making available lines of robots with a protection factor of IP65 and higher – that is, dustproof and resistant to water jets.
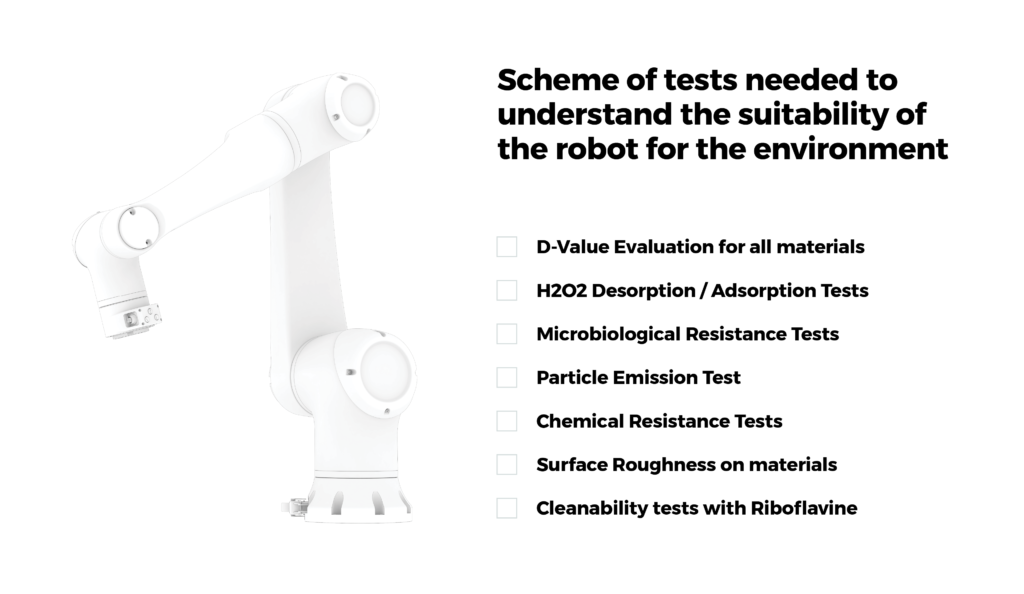
The robots are engineered to meet ISO 14644-1 and FS 209E standards and ensure high septicity, e.g., robot shells are constructed of materials that can be cleaned with isopropyl alcohol. The arms are subjected to rigorous tests to verify compliance with standards for chemical resistance, microbiological resistance, cleanability, and more. This commitment to maintaining sanitary standards is a testament to the industry’s dedication to quality and safety.
Scalability and flexibility
Changes in technology, products, and packaging require manufacturers to be more flexible. Therefore, an important feature of robotic systems used in this sector is their flexibility and scalability. Production can be easily adapted to changing market needs, allowing companies to manage production processes more flexibly and efficiently.
Cost reduction
The advantages mentioned above make it possible to reduce the involvement of specialized personnel in production-related activities. Automation saves money by reducing labor-related costs, waste, and the efficiency of production and logistics processes. Ultimately, this leads to increased competitiveness of pharmaceutical companies in the market.
Collaborative robots
Collaborative robots are an increasingly common solution in laboratories with limited as they can operate alongside lab workers without the need for fully separate automated workstations.. Laboratories perform many laborious and repetitive activities, such as pipetting, while being error-prone and high-precision.
Robots can precisely perform repetitive operations, supporting various laboratory activities such as counting, dispensing, mixing, quality control, and others. Growing popularity and user demands drive the development of solutions that allow the robot to be taught without programming – by observing the user’s actions.
Machine vision
A related topic to robots is machine vision. Vision systems, increasingly supported by artificial intelligence, can control the quality of the products being manufactured and help locate the products we will be processing—for example, trays of vials placed on a table.
Developments in artificial intelligence and three-dimensional image recognition are allowing robots to be used in new fields, such as picking unordered objects from a box.
Challenges
Designing robotic stations for the biotech and pharmaceutical environments presents many challenges. In these industries, diverse procedures with specific work environment requirements must be taken into account when designing the station.
Also, products come in a variety of forms, both liquid and solid. Liquids come in a wide range of packaging—from test tubes to vials, syringes to bags of considerable volume. Due to the often very high value of the substances used, working with these products must guarantee cleanliness and the lowest possible percentage of quality rejections.
Safety requirements
Robotic stations in regulated industries are required to meet the same rigorous safety standards as all other machinery within the European Union, as outlined by the Machinery Directive. When designing, a risk analysis must be performed regardless of whether the station is equipped with a collaborative robot.
Depending on the results of the analysis, it is determined whether the robot can work in the presence of humans, whether it must be enclosed, and how many and what products and process tools it can carry.
Collaboration
A4BEE, which operates in the biotechnology and pharmaceutical sectors, supports companies in designing and implementing appropriate technological solutions for manufacturing in regulated environments.
The significant number of standards in these industries and the multiplicity of solutions force collaboration with specialists in various fields – scientists, technologists, automation specialists, electricians, etc. Cooperating with experienced designers who will ensure that the designed stations are easy to clean and resistant and that the mechanisms used do not introduce particles into the monitored environment is indispensable.
Equally important is the collection of data that can be used to optimize production – with the increasing amount of data and integrations between robotic workstations and various systems such as MES, LIMS, as well as other equipment located in laboratories, or in a manufacturing plant’s machinery parks.






