
If you’re managing a lab in pharma, you already know this: regulations aren’t getting any looser, and the cost of manual work keeps climbing. What’s emerging here is a model that’s adaptable, scalable, and genuinely ready for whatever the future throws at it. For QC managers, the mandate is clear: build a quantified business case, lock in a roadmap that balances “quick wins” with long-term horizons, and embed compliance-by-design from day one. Done right, the digital lab is not just a technology upgrade – it is a strategic moat that turns regulatory rigor and operational excellence into a competitive advantage in an increasingly pressure-cooked pharmaceutical landscape. Let’s see how to make it happen.
Picture entering a next-generation QC lab – not a clipboard or USB stick in sight. Instead, the room buzzes with quiet efficiency: sensors capture real-time data, smart instruments sync effortlessly, and a unified digital platform ties it all together in perfect harmony. In our recent discussions with QC managers, we explored how to bring this level of intelligent connectivity to today’s labs. Drawing from our deep expertise in cutting-edge hardware and software, we zeroed in on the three dimensions that truly matter:
Business Impact: Quantifiable ROI, faster batch releases, reduced remediation costs, and minimized indirect losses.
Organizational Adoption: User engagement, simplified roles, and capability-building to ensure sustained value.
Technology Enablement: Robust, vendor-agnostic architecture that enforces ALCOA+ data (Attributable, Legible, Contemporaneous, Original, and Accurate) at every layer.
The big pain points: why change feels so urgent
Let’s face it, many pharmaceutical QC labs today are trapped in the clutches of disconnected devices, endless vendor versions, and paper-based processes. If you’re still relying on manual audits, switching USB drives from machine to machine, or hunting through reams of paper for an elusive measurement, you know the risks:
Disconnected devices: Dozens of vendors, each with their own quirks and costly add-ons.
Manual madness: No proper digital backup, administratively overwhelming user management, and a mountain of paper that grows with every shift.
Non-compliance nightmares: It’s not just a buzzword – manual work and lack of centralization equal high risk and high cost. ALCOA+ data approach is moving from guideline to Audit Requirement as both FDA and EMA inspectors now expect audit-ready electronic records that are Attributable, Legible, Contemporaneous, Original, Accurate – and Complete, Consistent, Enduring, Available.
As Lukas Paciorkowski, A4BEE Chief Strategy Officer says:
In a lot of cases the primary challenge we aimed to address is how to increase operational effectiveness and elevate compliance in labs that are currently largely paper-based and where equipment operates in a disconnected manner. And we clearly see that critical areas of pain points lie on a variety of layers…
…just to name most critical:
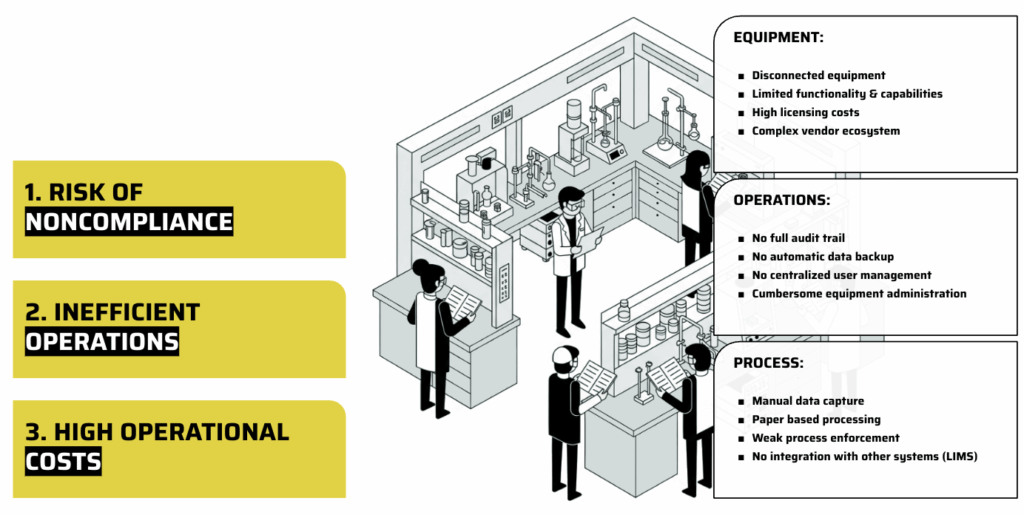
Equipment: Disconnected devices with limited functionality and high licensing costs, within a complex ecosystem of numerous vendors.
Operations: Lack of a complete, digitized audit trail, no automatic data backup (leading to manual methods like USB sticks), and cumbersome decentralized user management.
Process: Manual data capture, paper-based workflows, and weak enforcement due to disconnections, requiring substantial manual effort. These issues lead to non-compliance risks, inefficient operations, and high costs.
The 3 pillars of transformation
So what’s the prescription for lab modernization?
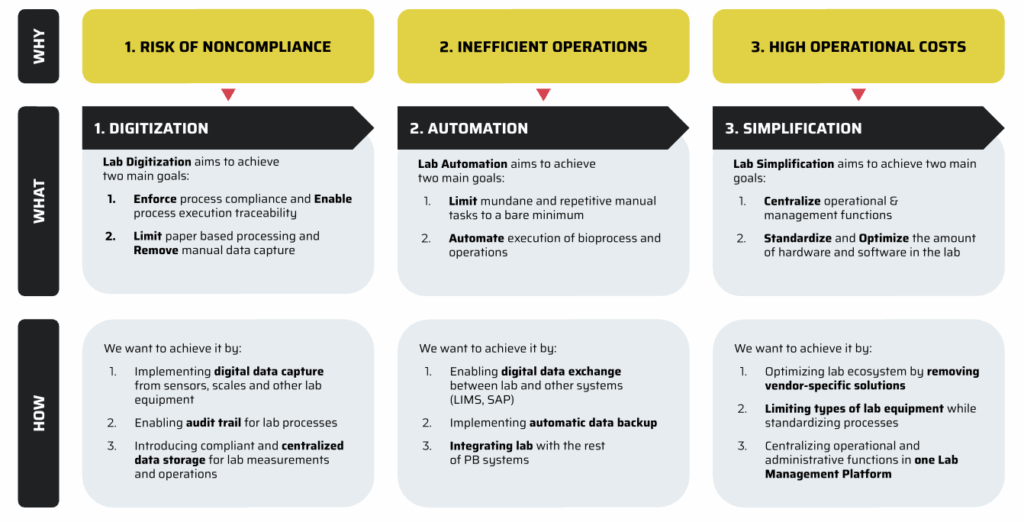
Digitization.
The first order of business is transforming how data is captured and managed. Instead of scribbles and scattered excel sheets, every instrument – scales, sensors, you name it – feeds data directly to a central system. The goal? No more missing audit trails, no more accidental “oops” data losses, and – best of all – one home for all your records.
Core action: Capture device outputs directly into a central platform with real-time audit trails.
Automation.
Why waste hours on repetitive tasks when machines can do the legwork? By integrating lab hardware with platforms that connect to systems like LIMS and SAP (and maybe even let you log in with your standard company password), mundane workflows dissolve. Think of it as self-checkout for your quarter-million-dollar lab kit.
Core action: Integrate edge gateways (like QB modules), LIMS, ELN, and SAP via standardized APIs.
Simplification.
Here’s where things really get interesting: imagine managing every device, user, and process from one place, regardless of how many vendors you’re juggling – or how many slightly different balance models you own. One interface. One way to work. Minimum chaos.
Core action: Standardize on a unified QB Control platform and consolidate hardware to multisensor gateways.
USE CASE
Working with a range of pharma and biotech clients, we’ve noticed a familiar pattern of QC challenges emerging again and again. To address them, we take a holistic approach, guided by a unified implementation vision and steps that we outline below.
CHALLENGE
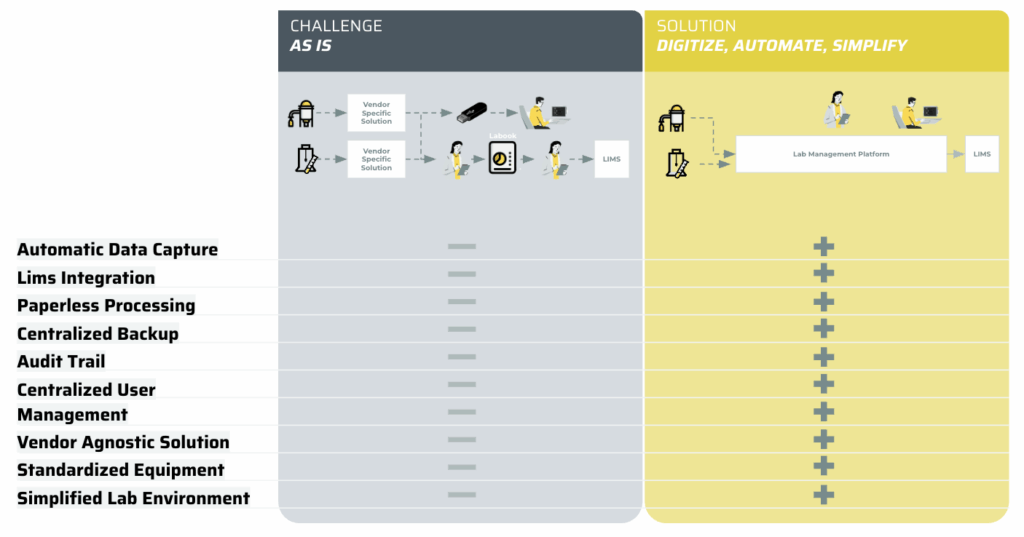
Current QC labs operate with disconnected, stand-alone devices, relying on paper records. Audit trails are incomplete, user management is manual, and compliance risks are high due to lack of central control. The main challenge that we tackle is often: How can laboratories that rely heavily on paper-based processes and disconnected equipment improve operational efficiency and enhance compliance?
SOLUTION
STEP 0. Define the implementation vision
We designed a holistic implementation vision grounded in in-depth interviews with stakeholders from QC, TD, IT, and QA, combined with findings from the feasibility study. The proposed solution addressed both immediate operational needs and long-term digital transformation goals.
STEP 1. Digitize and automate laboratory processes
We deploy an enterprise-grade lab management platform integrating directly with existing instruments via secure gateways. Operators authenticate with corporate credentials, execute tests through guided workflows, and capture results automatically. All data and operator actions were fully audit‑trail compliant, centrally archived, and synchronised with the Laboratory Information Management System (LIMS) in real time. Digital management of device and process reports eliminated manual uploads, local printers, and paper-based records — significantly improving data integrity, turnaround time, and traceability.
STEP 2. Simplify through vendor‑agnostic approach
The platform leverages direct integration with smart sensors, bypassing proprietary vendor controllers. This consolidated sensor data into a single system of record, reducing architectural complexity, operational overhead, and vendor lock‑in. By adopting a modular solution with open architecture, the lab gained the flexibility to integrate future equipment seamlessly, reduce maintenance costs, and maintain strategic control over its digital infrastructure.
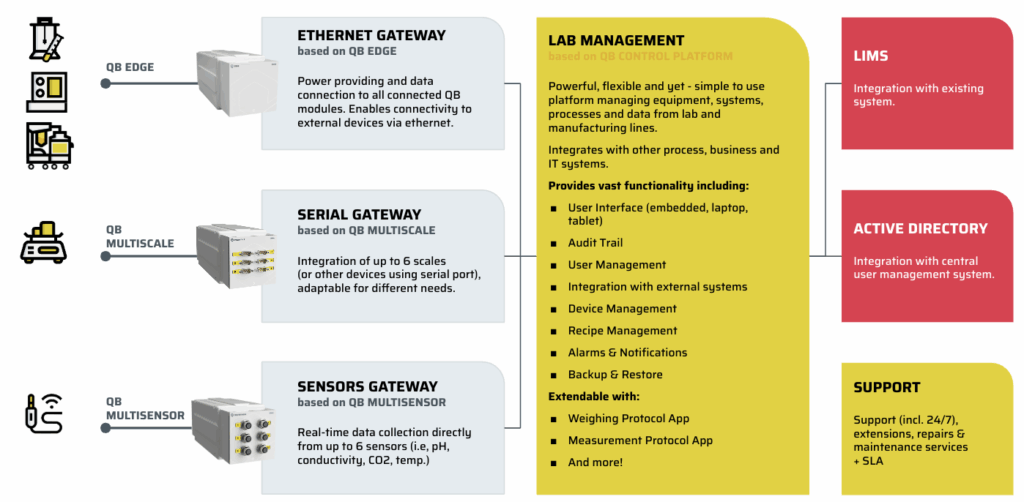
Technical core for the solution – QB Control platform
At its core we use QB Control by A4BEE – a central platform for managing laboratory equipment, processes, and data with full regulatory compliance.
Key capabilities included:
Real-time data acquisition from instruments via QB Edge Gateways and smart sensors, with seamless integration into the client’s LIMS and Active Directory.
Vendor-agnostic connectivity, eliminating the dependence on dedicated vendor controllers and ensuring scalability and flexibility in integrating new instruments.
End-to-end data integrity through automated audit trails, secure data storage, and structured workflows that meet regulatory and quality assurance requirements.
Digital SOPs guiding operators through standardized, stepwise workflows, reducing errors and training time.
Centralized management of users, devices, methods, and alarms, improving operational control and reducing variability.
By combining modular, open-architecture hardware components (QB modules) with an enterprise-grade software layer, we enabled the lab to transition from fragmented, manual processes to a fully connected, simplified, and compliant operating environment — without costly or complex custom integrations.
As Jacek Fischbach, A4BEE Delivery Executive says:
It should be noted that unlike many off-the-shelf SaaS offerings, this solution runs entirely within the client’s infrastructure, ensuring maximum control and security – though the platform can also be installed in the cloud where desired.
OUTCOME
Compliance, efficiency, and sanity restored
This is more than a new look for the lab; it’s a new backbone that supports compliance at every step, slashes costs, and makes growing your QC operation far less scary. Data integrity is enforced, user management is unified, audit trails are complete, and – crucially – IT headaches shrink instead of multiply. Centralized control doesn’t just make things easier; it makes them safer.
Across dozens of pharma plants, data show a consistent pattern: QC labs that move decisively beyond paper not only slash costs but dramatically de-risk compliance exposure. With investment paybacks well under five years and tooling ecosystems maturing fast, the question is no longer if but how soon you will accelerate your lab’s digital evolution.
OPTIMIZED OVERALL LAB COSTS
ENSURED ROBUST COMPLIANCE
ENHANCED OPERATIONAL EFFECTIVENESS






