Sartorius bioreactors retrofit with flexible and plug and play control
Industry
Pharma / Biotech
Scope
IT/OT Integration / Custom Control Software / Hardware Retrofitting / MTP Standard
Timeframe
3 months
-
40%
faster calibration
-
60%
reduction in manual data entry
-
3
months of complete transformation to an open platform
01
CLIENT
A leading pharmaceutical producer and CDMO (Contract Development and Manufacturing Organization) develops and commercializes medicinal products and related healthcare solutions, combining research and development capabilities with manufacturing expertise. Its portfolio includes therapies used in long-term patient care as well as complementary products. The company operates modern manufacturing and quality control facilities, enabling processes that comply with regulatory requirements and GMP standards.
02
BUSINESS NEEDS
The client needed to integrate laboratory bioreactors into a single, centralized control system to eliminate fragmented data silos. The requirement was to manage a diverse laboratory and production setup, consisting of four control towers for glass-vessel bioreactors (ranging from 1L to 5L) and a large-scale 70L stainless steel bioreactor tower.
Beyond basic control, the goal was to extend the systems' capabilities by implementing advanced data acquisition, centralized recipe management, and sophisticated process analytics - features that surpassed the native functionality of the original Sartorius-based setup.
03
CHALLENGE
To help our client achieve its goals, we overcame the following challenges:
-
Vendor Lock-in
Dealing with closed PLC controllers where the original source code was inaccessible for modifications. -
Rigid Hardware
Working with proprietary hardware that offered no native options for reconfiguration or expansion. -
System Interoperability
Bridging the gap between legacy industrial PLC logic and the modern QB digital platform. -
Sensor Standardization
Harmonizing various proprietary sensor connectors and communication protocols into a unified interface.

04
SOLUTION
We started with the technical retrofit of the bioreactor control layer, replacing restrictive legacy hardware with an open, scalable architecture. To streamline operations, we implemented a centralized system where four control towers for glass-vessel bioreactors (1L–5L) and one 70L stainless steel bioreactor tower were all integrated into a single QB Edge gateway.
Hardware integration
- PLC Modernization Replaced the existing, closed PLC controller with a new, open unit to allow for custom logic implementation with vendor agnostic approach.
- Edge Connectivity Integrated QB Edge to serve as the single gateway between the production floor and the digital platform for all connected units.
- Sensor Refurbishment Replaced Hamilton VP8 sensor connectors with standard M12 connectors and routed liquid level/foam sensors via relays to a new I/O coupler.
- Smart Device Networking Connected Hamilton measurement devices to an IOLAN server to enable network-based data access.
Building on this open foundation, we implemented a modular software architecture based on MTP standards to bridge the gap between local process control and the centralized digital platform. Client was equipped with new previously not available capabilities like Audit Trail, Recipe Management, User-Customizable P&ID, User Management with Remote Access to the system.
Software Integration
- MTP-Based Programming Quickly programmed the new PLC using A4BEE’s proprietary MTP library, ensuring the code is modular and future-proof.
- Advanced Process Logic Replicated and optimized Sartorius cascade regulator logic within MTP services for precise process supervision.
- Data Orchestration Configured a proprietary OPC UA connector for seamless data transmission via the MQTT protocol.
- QB Control Deployment Installed and configured QB Control on the Edge device, enabling centralized management of the bioreactor's pumps, pH, and DO sensor calibrations.

This bioreactor integration was about transforming a restricted 'black box' into a high-performance, open asset. By replacing the locked controllers, we successfully removed the vendor barriers that had been limiting the client’s operations. Implementing the QB Control platform and MTP standards gave them full ownership of their process logic, allowing them to scale their research independently, without being tied to a single provider’s roadmap.
Łukasz Paciorkowski
CEO
Technology used
05
OUTCOME
The implementation transformed the laboratory's capabilities, providing a unified and transparent environment for complex bioprocessing.
- Centralized Supervision Total control over all bioreactors from a single interface, including advanced recipe execution.
- Ownership of Logic Elimination of vendor lock-in, allowing the client to modify and evolve their own process control strategies.
- Enhanced Data Acquisition Real-time data streaming and historical logging, enabling deeper process insights and better quality control.
- Scalable Infrastructure A modular "blueprint" that allows for the easy addition of new bioreactors or sensors regardless of the original manufacturer.
- Cost Efficiency Significant reduction in licensing overhead and long-term TCO (Total Cost of Ownership) by moving to an open, hardware-agnostic architecture.
- Unrestricted Data Access Complete and unlimited ownership of generated data, allowing for free-flowing information across the entire organization without additional per-user or per-volume fees.
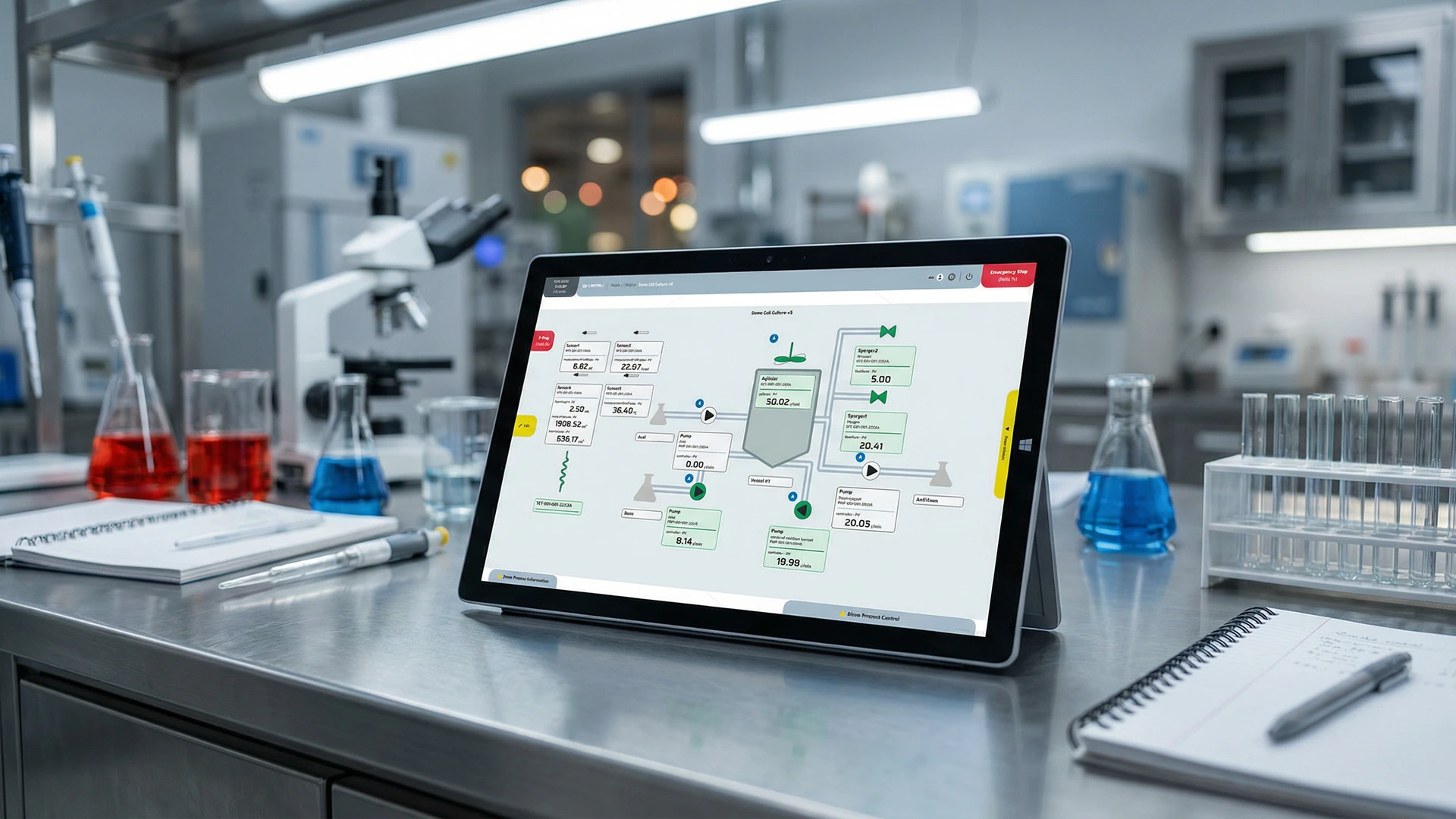

06
IMPLEMENTED SOLUTION
-
Controller Replacement
Swapping proprietary PLCs for open-standard hardware to enable full access to the control logic. -
Standardized Interfacing
Converting proprietary Hamilton VP8 connectors to M12 to simplify maintenance and hardware replacement. -
Service Configuration
Implementing MTP services specifically designed for cascade regulation, ensuring stable and repeatable bioreactor environments. -
Calibration Management
Integrating automated calibration workflows for pumps and sensors directly into the QB Control interface.
-
40%
faster calibration
-
60%
reduction in manual data entry
-
3
months of complete transformation to an open platform